potassium iodide conductivity
Platinous iodide I2Pt CID 139965 - structure chemical names physical and chemical properties classification patents literature biological activities safety. The compound KAg 4 I 5 has an exceptionally high ionic conductivity for a solid reaching 031 ohm 1 cm 1 at the incongruent melting point.
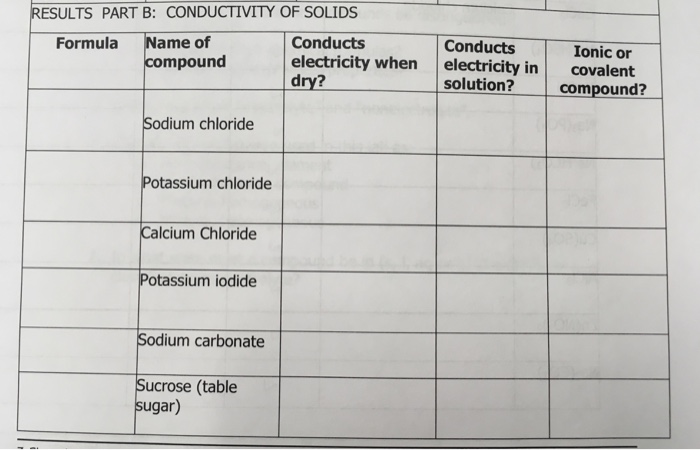
Solved Results Part B Conductivity Of Solids Conducts Chegg Com
Thermodynamic data derived from emf.
. Temp F µScm Acetaldehyde 59 17. The region A can be given a conventional interpretation in terms of m. The solubility of Ba 2 in KI is much higher than that of Cd 2 Pb 2 and Ca 2.
500 mL 1 L 4 L. Two regions A and B corresponding to different activation energy of conductivity are identified. We find that the surface states are passivated apart from the modified lattice structure.
Potassium iodide solution is ionic making it electrically conductive. It can also conduct electricity when molten but this is due to the potassium ions rather than the iodine ions. Electrical conductivity is denoted by the Greek letter σ sigma and its measured.
It also can conduct electrical energy when moltenhowever that is as a result of. In some radiation emergencies usually nuclear power plant accidents radioactive iodine may be released into the environment and enter the body through breathing or eating it. A large contribution to the conductivity by the anion vacancies is observed in pure KI at high temperatures.
Potassium iodide solution is ionic making it electrically conductive. Conductivity Chart of Liquids conductivity too low for mag Low conductivity appl. At lower temperatures the conductivity of KI is suppressed by the CO32-impurity.
The ionic transference number is in the range of 095 to 098. Potassium Iodide 5 644 33800 10 68000 20 146000. The solid salt of potassium and iodine potassium iodide KI conducts poorly as do all salts which are ionic compounds.
However when in a solid dry form the compound does not conduct electricity. Solid potassium iodide which is an ionic compound cannot conduct electricity because although the ions are charged they are not free to move around when. The electrical conductivity of pure KI and CdI₂-doped KI has been studied in the temperature range 200 to 23C.
A range in which the specific conductivity increases 10000 times. This key property can be increased by adding metallic or ceramic. Please contact the SmartMeasurement factory for assistance.
The direct current conductivity of DMSO KI solutions was determined with the use of the impedance spectroscopy. If a particular chemical is not listed or if you would like application assistance with an electromagnetic flowmeter application please feel free to contact SmartMeasurement TM s US. The values obtained for various energies of activation in KI crystals are Ec 072002 ev for the migration of a cation vacancy EA 150005 ev for the.
However when in a strong dry type the compound doesnt conduct electrical energy. Potassium iodide KI is a type of iodine that is not radioactive and can be used to help block one type of radioactive material radioactive iodine I-131 from being absorbed by the thyroid. However when in a solid dry form the compound does not conduct electricity.
Potassium iodide is a component in the electrolyte of dyes sensitized solar cells DSSC along with iodine. Ionic conductivity of pure and doped KI is measured in the temperature range 200 to 700c. 228 rows Conductivity values displayed in yellow are questionable.
The conductivity reaches 366. The conductivity reaches 366 10 4 Scm at ambient temperature. Offices at 1-866-404-5415 for assistance.
It can also conduct electricity when molten but this is due to the potassium ions rather than the iodine ions. The current is carried by the Ag ions. A significant increase in the conductivity and radiative efficiency is achieved under such conditions.
Name by Wt. The measurements were performed in. Measurements indicate that the formation of KAg 4 I 5 from K 2 AgI 3 and AgI is accompanied by an entropy gain implying an unusually high degree.
Potassium Chloride Conductivity Standard 100 μScm at 25C Ricca Chemical Structure Search. Here we systematically investigate the regulatory mechanisms of the performance of perovskites by exploiting potassium iodide KI doping. Crystals doped with CO32-show an increase in conductivity at high temperatures owing to the increase in anion vacancy concentration.
Have determined with a fair degree of accuracy the conductivity of solu tions of potassium iodide in liquid iodine at temperatures from 120 degrees to 160 degrees and in concentrations ranging from a few thou sandths normal up to 10 times normal. Print Potassium Chloride Conductivity Standard 100 μScm at 25C Ricca Chemical. The activity of a salt in an electrolyte has a direct impact on the performance of electrochemical cells because it can affect both the potential between phases and transport through the electrolyte.
Click to view available options Quantity. Potassium iodide resolution is ionic making it electrically conductive. Thermal conductivity of a typical unfilled epoxy system has a very low value of 014 WmK.
Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature
The Effect Of Potassium Iodide On The Viscosity Of Vegetable Oil N N Dimethylformamide Solvent At Different Temperatures Springerlink
Solutions Of Potassium And Iodide In Water And Solution Of Lead Nitrate In Water Being Mixed Together Turning Yellow And Forming A Lead Iodide Stock Photo Alamy
Polymers Free Full Text Role Of Mg No3 2 As Defective Agent In Ameliorating The Electrical Conductivity Structural And Electrochemical Properties Of Agarose Based Polymer Electrolytes Html
Pdf Ionic Interaction Of Potassium Iodide In Edible Oils Dmf System By Viscosity Method Semantic Scholar
Why Does Potassium Iodide Solution Conduct Electricity
Why Does Potassium Iodide Solution Conduct Electricity
Pdf Electrical Conductivity Of Potassium Salt Dimethylsulfoxide Water Systems At Different Temperatures Semantic Scholar
Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature
Quenching Of Fluorescence Intensity Of Dox Using Potassium Iodide Download Scientific Diagram
Why Does Potassium Iodide Solution Conduct Electricity
Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature
Ionic Conductivity Variation With The Alkaline Iodide Salt Mi Lii Download Scientific Diagram
Detection Of Radical Activity Using A Potassium Iodide Ki B Download Scientific Diagram
Ionic Conductivity And Dielectric Constant Data Of A Agarose With Ki Download Scientific Diagram
Pdf Solubility And Density Of Potassium Iodide In Binary Ethanol Water Solvent Mixture At15 K Ramesh Pawar Academia Edu
Potassium Iodide Conductivity Youtube
Temperature Dependence Of The Ionic Conductivity Of The Gel Polymer Download Scientific Diagram
Pdf Electrical Conductivity Of Potassium Iodide Between 200 C And Room Temperature
Comments
Post a Comment